
A Bright Future Ahead
Portable Spectral Services Announces Exciting Management Transition
What is Pewter?
Pewter is a malleable metal alloy with traditionally 85-99% tin, with the remainder 1-4% consisting of copper (hardener) and lead (lower grade pewter). Pewter is similar in appearance to silver, as it is shiny, and will oxidise to a dull grey over time if left untreated. Few pewter artefacts have survived the centuries as any pewter that was damaged or worn would have be melted and recycled.
Types of Pewter
The Worship Company of Pewterers controlled pewter constituents in England in the 15th century. This company laid down the composition of the pewter alloy, originally in two grades. The first was known as “fine metal” or “eating ware,” and was used for tableware. This was made from 96-99% tin, and 1-4% copper. The second type, known as “trifling metal” or “trifle” was used for hollowware (Holloware is metal tableware such as teapots, water jugs, sugar bowls etc) and is usually made with 92% tin, 1-4% copper and up to 4% lead.
In the 16th century, an additional type was created called “lay” or “ley” and was used for items not in contact with food or drink. Lay usually contained up to 15% lead. These three types of alloys were used with little variation until the 20th century.
How has Pewter been used?
Pewter has been referred to as “the poor man’s silver.” It was first used around the beginning of the Bronze Age, with the earliest known piece found in an Egyptian tomb from 1450 BC.
Pewter was very common during the Middle Ages and was used extensively until the development of pottery and glassmaking during the 18th and 19th centuries. An irreversible shift occurred when pewter, once the chief material used for plates, cups and bowls in daily life was replaced by the mass production of pottery, porcelain and glass products. By the 1850s pewter had largely disappeared. Later in the 20th century it made a resurgence in popularity, due to the introduction of the Art Nouveau styles of Liberty’s Tudric range.
In the 1950s and 1960s centrifugal casting was developed and this new technology led to a boom in the popularity of pewter in jewellery and artware.

Image 1: Pewter Jugs used in analysis
A staff member was gifted two vintage Selangor Pewter jugs (as shown by the stamping on the base). Both were tested using a Bruker Titan S1 800 using the restricted materials calibration.

Image 2: Results screen from S1 Titan using RoHS Calibration
The results

Spectra 1: Pewter Jug 2 side analysis

Spectra 2: Pewter Jug 1 handle analysis
What health risks are there?
If there is a possibility that a pewter item in your possession contains lead it is strongly advised that you no longer use the item for any purposes that where your body comes into contact with the item (e.g. cups, plates, jugs and jewellery). This is advised because the lead could be leaching out of the metal and there are many health concerns that stem from coming into contact with lead over a prolonged period of time.

Spectra 3: Pewter Jug 1 lid analysis
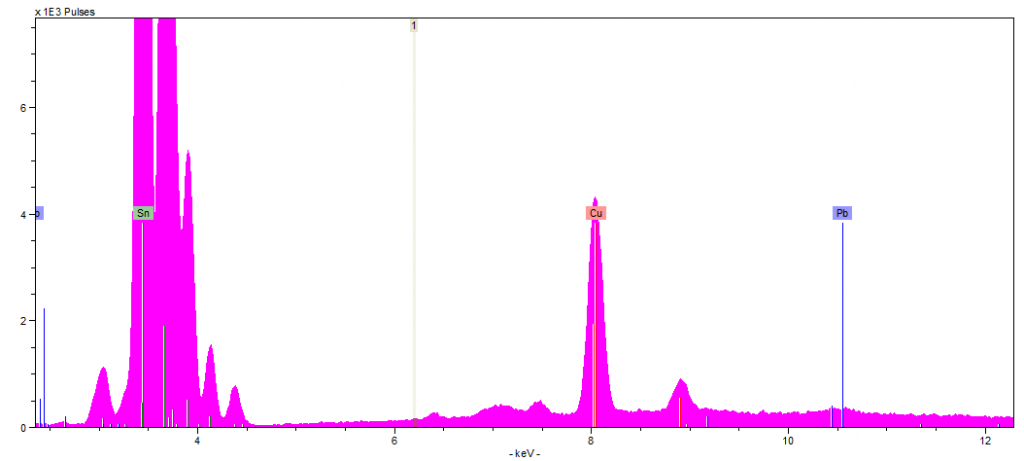
Spectra 4: Pewter Jug 1 bottom analysis
Fun fact: If Pewter contains lead it will get darker with age and will become more difficult to buff to its original shine and colour.
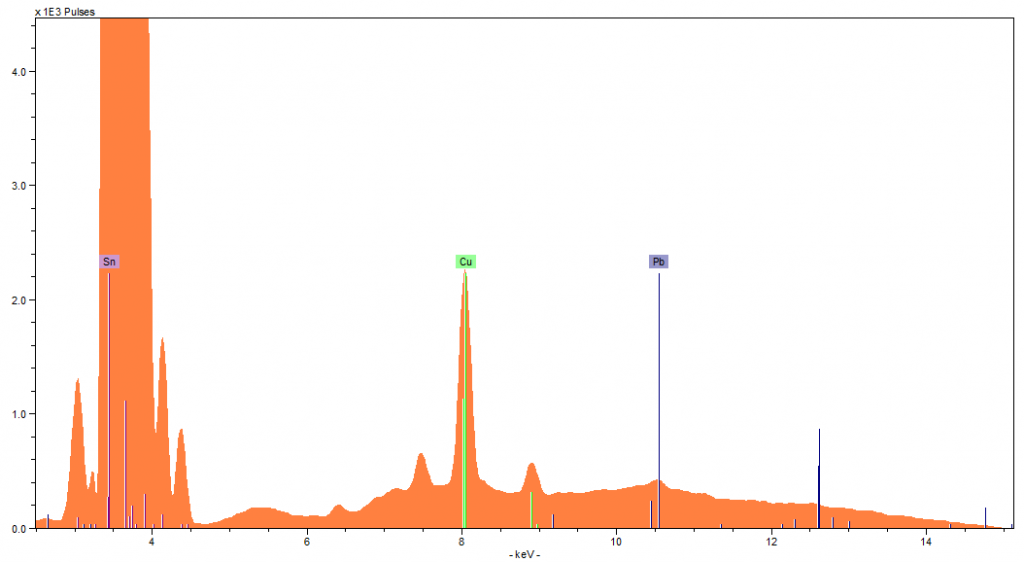
Spectra 5: Jug 2 side

Spectra 6: Jug 2 side
Discussion:
The recommended value of lead should be below 1000 ppm for any item that a person comes into contact with on a daily basis. Anything above this level is considered unsafe, and should be avoided for its intended use, especially if in contact with the human body.
Jug one contained lead values of 1580 ppm on the side (Spectra 1) and 2404 ppm on the lid (Spectra 2). Both values are higher than the recommended 1000 ppm level. The differing lead concentrations within the jug could be from temperature as this jug has been previously used. Spectra 3 was taken from the bottom of the pewter jug and passed the RoHS, highlighting that the lead values vary across the different surfaces of the jug.
Jug two contained lower levels of lead, with 1071 ppm in the side (Spectral 4), and 1331 ppm in the lid (spectra 5). Although both readings were lower, they were still above the recommended amount. The wooden handle of the jug passed the RoHS scan, so there was nothing harmful in the coating on top of the wood.

Conclusion:
Both pewter jugs came back with elevated levels of lead. As lead was detected in quantities above the safe limit (recommended levels are <1000ppm). The jugs will therefore not be used for used for its primary purpose of storing liquids for drinking purposes.
Instead our staff members will have to be creative and find an alternative use for them. A vase perhaps?
Further Reading:
If this has sparked some interest, read further about RoHS here:
http://www.rohsguide.com/rohs-substances.htm
More about pewters here:

Portable Spectral Services Announces Exciting Management Transition

Our tool introduces uXRF (micro-X-ray fluorescence) scanning technology to RC chip analysis, enabling rapid, non-destructive, and quantitative analysis of major, minor, and trace mineral phases.

Automated micro-X-ray fluorescence (micro XRF) technology emerges as a powerful tool to rapidly and accurately capture the mineralogy of rock chip, RC and AC samples.

Findings of an ongoing regional evaluation study over concealed Proterozoic lithologies known to host magmatic nickel sulphides with potential to host other base-metal, gold and rare earth elements (“REE”) systems within the Fraser Range, Western Australia.

Findings of an ongoing regional evaluation study over concealed Proterozoic lithologies known to host magmatic nickel sulphides with potential to host other base-metal, gold and rare earth elements (“REE”) systems within the Fraser Range, Western Australia.
Findings of an ongoing regional evaluation study over concealed Proterozoic lithologies known to host magmatic nickel sulphides with potential to host other base-metal, gold and rare earth elements (“REE”) systems within the Fraser Range, Western Australia.
