
A Bright Future Ahead
Portable Spectral Services Announces Exciting Management Transition
Lithium-ion batteries come in a range of shapes, sizes and compositions, and these batteries are revolutionizing the way we produce, conserve, and use electricity. Since the late 1990s, advances in lithium-ion battery technology has been driven by the ever-increasing demand for mobile phones, laptops and other portable electronics. This technology has now been comprehensively applied to the automobile industry and the use of lithium-ion batteries has enabled electric cars to become a beacon of the future – green and reliable. Here we will look inside a lithium-ion (NCA) battery that has been provided by Talga Resources to understand the internal structure and chemical composition of this revolutionary battery (Fig. 1a).
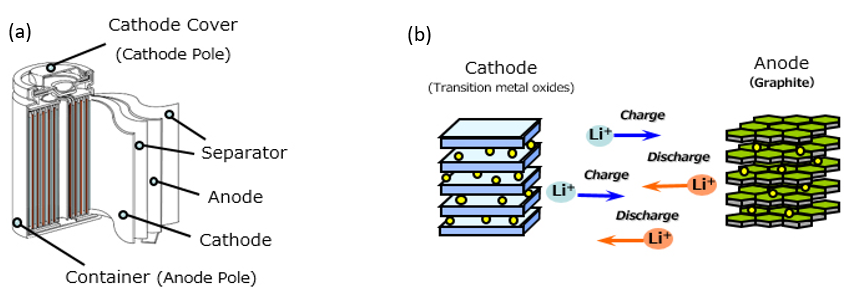
Figure 1 (a) Schematic of the internal structure within a lithium-ion battery and (b) the transfer of Li-ions during discharge and charge.
Image from here.
Electric car manufacturers use different cathode chemistries for their lithium-ion batteries. Tesla uses NCA (Nickel, Cobalt, Aluminum) chemistry, while other manufacturers more commonly use NMC (Nickel-manganese-cobalt) chemistry. The use of NCA chemistry is thought to improve energy density, increase charging performance, and provide longer cycle life. The battery in these cars are made up of several thousand of these small cylindrical cells (Fig. 1a).
The Bruker M4 TORNADO is a non-destructive, high-resolution micro- X-ray fluorescence instrument that can be used to produce detailed elemental maps of a sample area, using a spot size of 25 microns. The battery was analysed under vacuum (20 mbar) over an area of 6.5 by 2 cm at 30 micron resolution (50kV, 400uA). The Bruker M4 TORNADO can detect and quantify elements from sodium (Na) to uranium (U) and has two detectors to improve accuracy and acquisition time.

Figure 2: Internal structure of an NCA battery with combined element maps showing the Ni-Co cathode (yellow), anode current collector (blue).
An NCA battery generates power through chemical reactions between the anode and cathode. Within the core of the NCA battery we can see the Ni-Co cathode (on an Al current collector) and graphite anode (on a Cu current collector) (Fig. 2). These electrodes (cathode and anode) have a layered internal structure with a separator between the electrodes composed of a very thin sheet of microperforated plastic (Fig. 2). The lithium-ions located between the layers in electrolyte move back and forth between the two electrodes within the battery when the battery discharges and charges (Fig. 1b). The scan shows that the internals of the battery are not symmetrical, as the gaps between the cathode and anode in the bottom of the battery are wider than the top (Fig. 2). Additionally, the concentration of Cu in the anode and Ni in the cathode varies across the battery (Fig. 3), while the Co content remains consistent. The changes in the Cu content in the anode may be attributed to either a variation in the Cu metal abundance, or the surface of the cut battery not being completely flat.
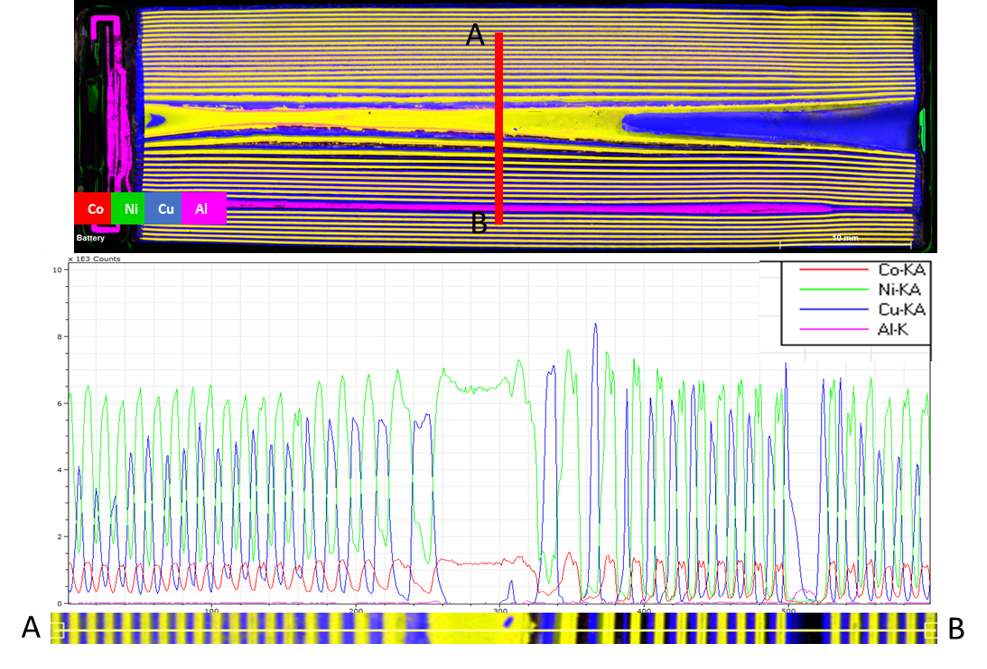
Figure 3: A line scan across the battery showing the relative distribution of Co (red), Ni (green), Cu (blue) and Al (pink) across the battery.
The opportunity to view the internals of the NCA battery provides us with insight into the composition and configuration of these revolutionary batteries. The use of micro-XRF technology enabled us to rapidly scan the battery before it had extensively oxidized and produced high resolution element maps providing remarkable detail on the internal structure.
As the experts and innovators in spectral technology, Portable Spectral Services provides a distinctive voice for spectral equipment. Founded on a strong commitment to research and development, Portable Spectral Services is always evolving to meet dynamic market demands, providing an array of comprehensive services. Portable Spectral Services (formerly Portable XRF Services) was established in Perth, Australia in 2010.
Talga Resources Ltd (ASX:TLG) is a highly integrated developing producer of lithium-ion battery anode products, technologies and industrial graphene additives. The Company’s main focus is building an integrated graphite anode facility in Sweden running on 100% renewable electricity, to produce ultra-low emission coated anode for greener Li-ion batteries. Customer tests confirm the high performance of Talga’s products, and qualification processes with battery manufacturers and automotive OEM’s is underway targeting commercial production in 2023.
For more information on micro-XRF spectroscopy visit www.microxrf.com.au/.
Visit our YouTube channel, Professor Spectrum to watch live micro-XRF scans and instructional videos.
If you are interested in having your own sample analysed by micro-XRF, contact Portable Spectral Services p: 08 9321 2830 e: [email protected]
Product information on the Bruker M4 TORNADO micro-XRF.

Portable Spectral Services Announces Exciting Management Transition

Our tool introduces uXRF (micro-X-ray fluorescence) scanning technology to RC chip analysis, enabling rapid, non-destructive, and quantitative analysis of major, minor, and trace mineral phases.

Automated micro-X-ray fluorescence (micro XRF) technology emerges as a powerful tool to rapidly and accurately capture the mineralogy of rock chip, RC and AC samples.

Findings of an ongoing regional evaluation study over concealed Proterozoic lithologies known to host magmatic nickel sulphides with potential to host other base-metal, gold and rare earth elements (“REE”) systems within the Fraser Range, Western Australia.

Findings of an ongoing regional evaluation study over concealed Proterozoic lithologies known to host magmatic nickel sulphides with potential to host other base-metal, gold and rare earth elements (“REE”) systems within the Fraser Range, Western Australia.
Findings of an ongoing regional evaluation study over concealed Proterozoic lithologies known to host magmatic nickel sulphides with potential to host other base-metal, gold and rare earth elements (“REE”) systems within the Fraser Range, Western Australia.